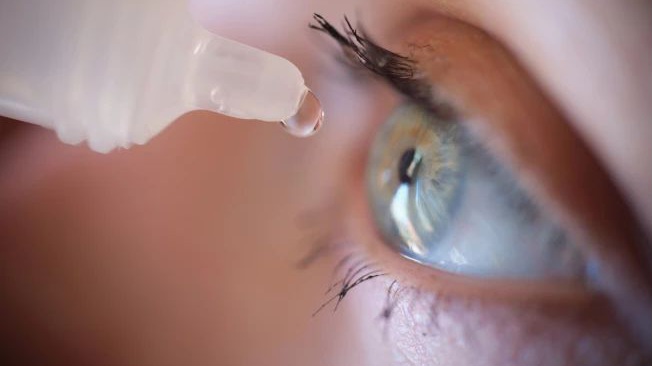
FDA approves first eye drop without side effects to treat presbyopia
The US Food and Drug Administration (FDA) has approved the first eye drop called VIZZ that improves near vision in people with presbyopia by changing the focus of the pupil without side effects.
These drops, produced by Lenz Therapeutics, provide clearer vision for activities such as reading or working on the computer for up to 10 hours as a non-surgical alternative to glasses or contact lenses.
According to Popular Science, it works using the active ingredient aceclidine, which creates a “pinhole” effect by contracting the iris sphincter muscle and increases the eye’s depth of focus without negatively affecting distance vision.
Presbyopia, which is caused by a decrease in the flexibility of the eye lens with age, affects more than 1.8 billion people in the world.
Used once a day, Vis takes effect in less than 30 minutes and improves near vision for up to 10 hours.